Vista Research Group, Inc. provides two types of research to addiction treatment and behavioral healthcare facilities:
- INSIGHT Addiction™, INSIGHT Detox™, and INSIGHT Behavioral™ monitor patients while they’re in treatment and provide real-time results to their clinicians in easy-to-read graphs to inform clinical care.
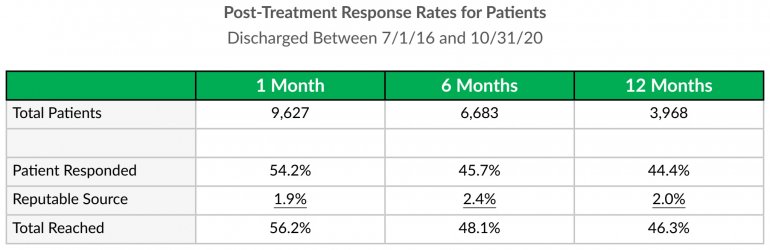
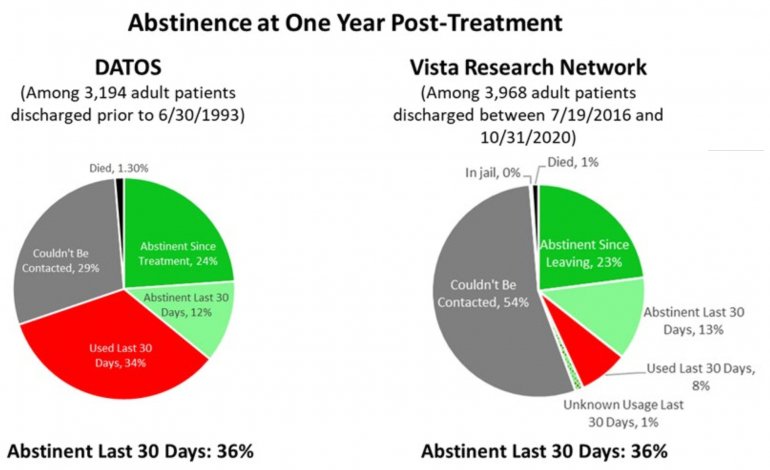
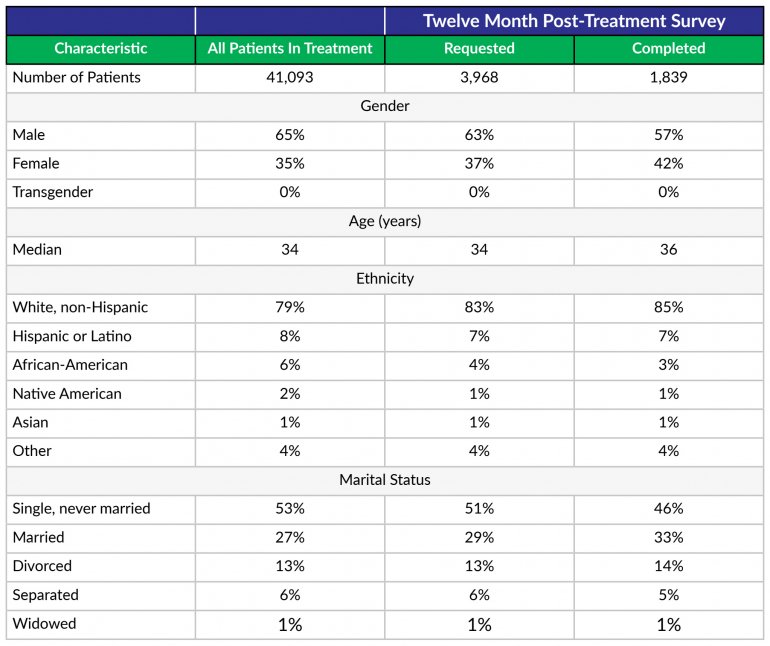
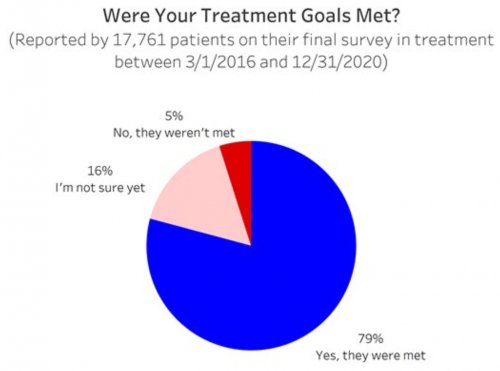
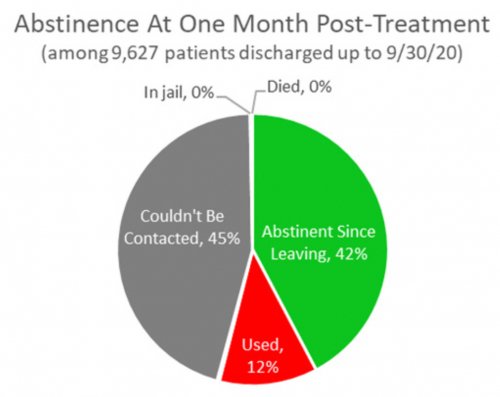
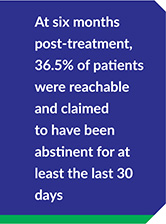
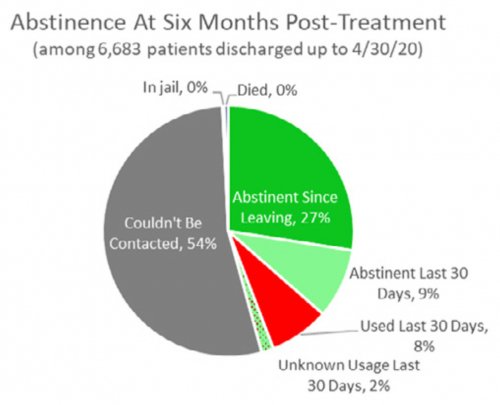
- RECOVERY 20/20™ follows up with patients at 1 month, 6 months and 12 months post-treatment to confidentially learn how they’re doing and whether they’ve been able to meet their drug- and alcoholusage goals post-treatment.
All Vista clients use INSIGHT™ products to monitor their patients during treatment. The majority of Vista clients also use RECOVERY 20/20™ to follow up with at least a random selection of their patients after discharge.
All INSIGHT and RECOVERY 20/20 data is patient-reported. In most cases, patients complete online surveys themselves using a tablet, cell phone or computer. Occasionally, patients are asked the survey questions by an outcomes researcher who types their answers directly into the survey tool during the interview.
Enrollment in Research: Patients entering treatment start the enrollment process by submitting a HIPAAcompliant online permission form that gives Vista Research Group permission to collect their personal health information, share their results with their treatment center, and contact them both during and at one month, six months and twelve months after treatment. In most states, a parent or legal guardian must also provide permission for adolescents to be enrolled in the research. Patients can opt out of the research at any time.
Collecting Intake Data: Once patients have given their permission for the research, they’re asked to complete an intake questionnaire that does the following:
- Collects basic demographic and health information
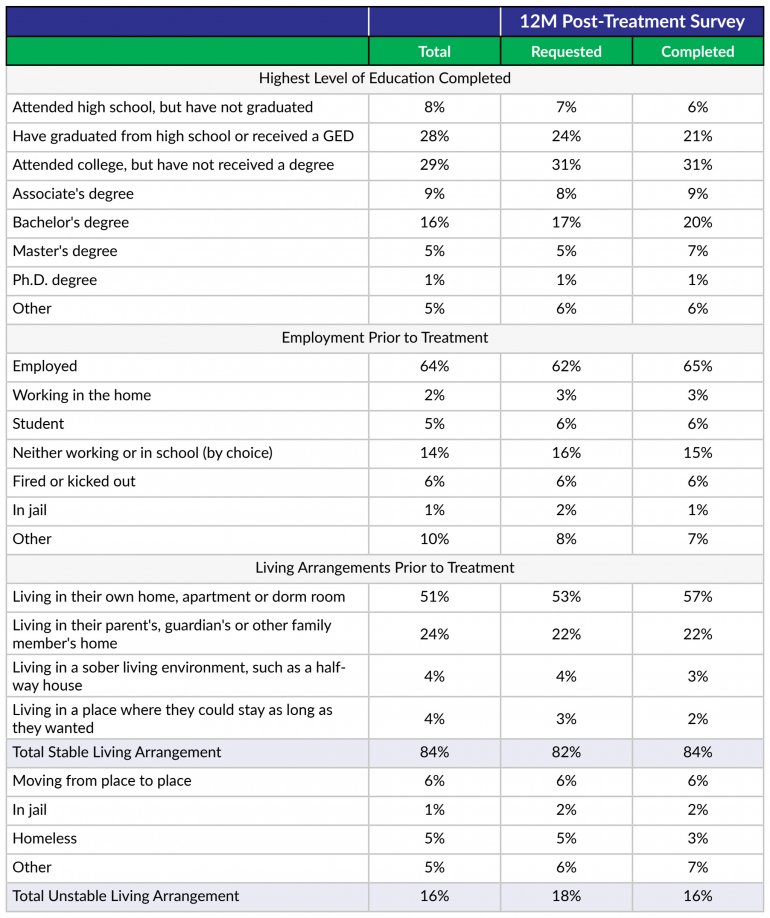
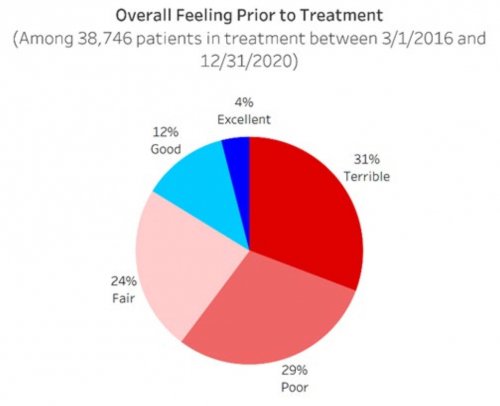
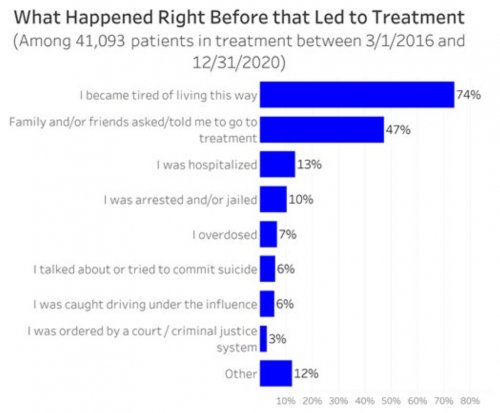
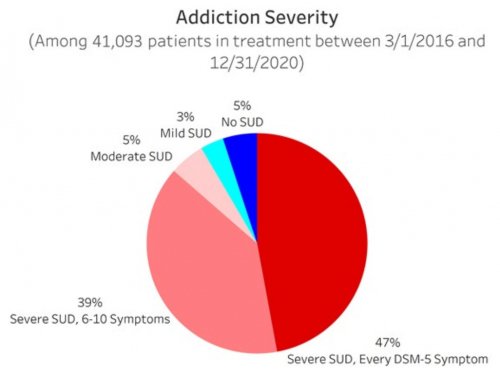
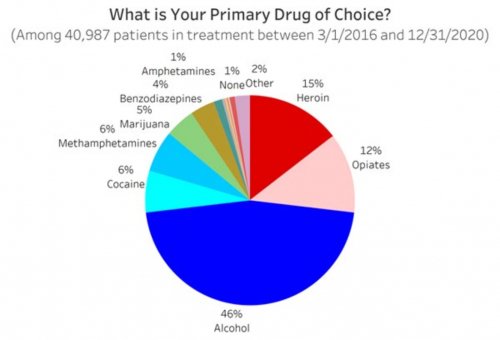
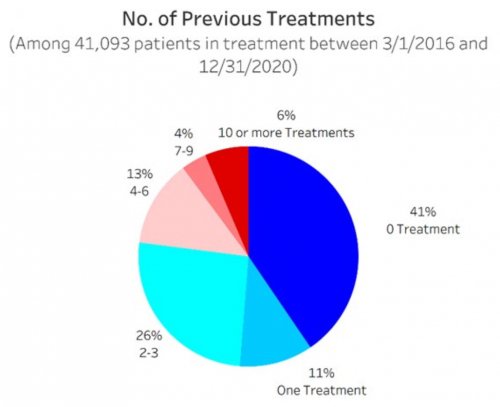
- Determines their primary drug of choice, quantifies their drug and alcohol usage pre-treatment, assesses the severity of their addiction, and identifies the factors that led them to enroll in treatment
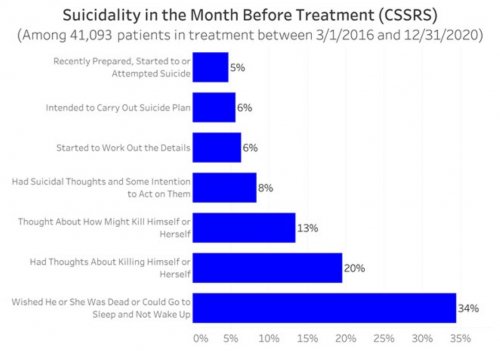
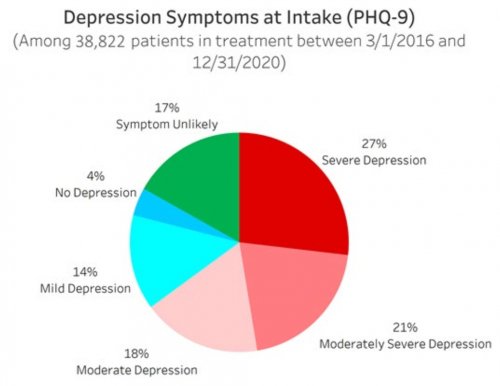
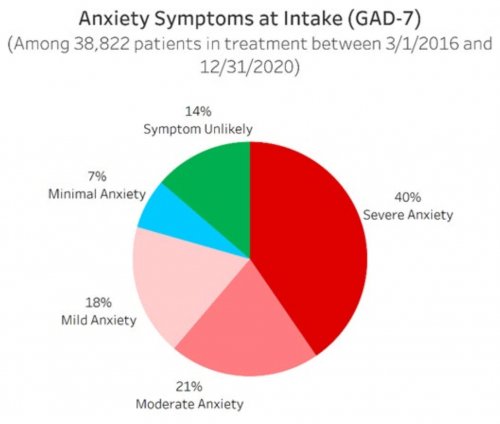
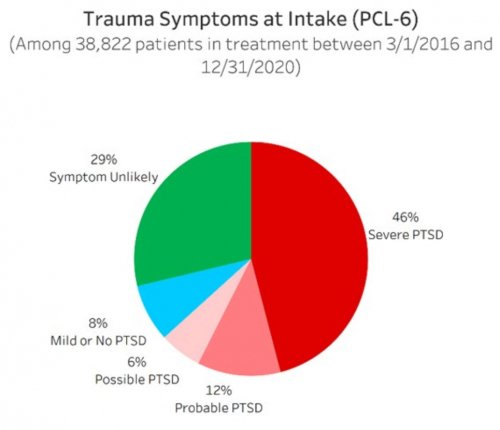
- Screens patients for common mental disorders and self-harming thoughts or behaviors using simple yes/no questions that have been academically validated to predict whether a patient is likely to be experiencing that disorder
- Uses academically-validated instruments designed for patient self-report to assess the severity during the 30 days prior to treatment of the common mental disorders and self-harming thoughts or behaviors that patients screened positively for
The intake questionnaire typically takes patients 15 to 25 minutes to complete online. At the center’s discretion, patients in acute withdrawal from drugs or alcohol can complete the intake survey in two sittings. In this case, questions about co-occurring disorders are not asked until the second part of the intake questionnaire and the patient’s emotional or physical withdrawal symptoms are monitored during the beginning of treatment instead of their co-occurring disorder symptoms.
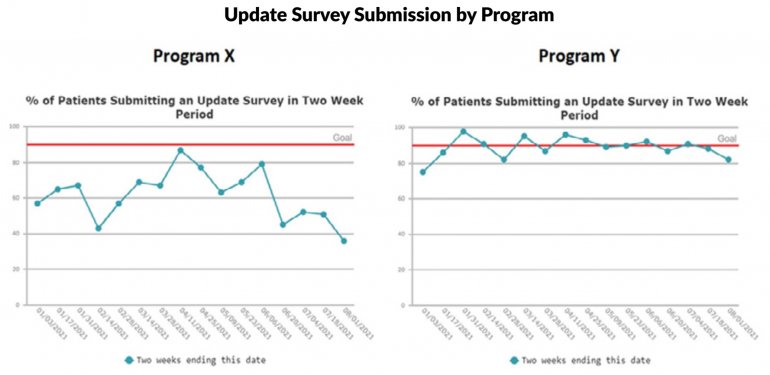
Monitoring Patients During Treatment: Patients update their clinicians on how they are feeling throughout their time in treatment by completing 2- to 3- minute surveys that have been customized to ask only the questions that are relevant to that patient. Vista recommends that patients provide update surveys on a weekly basis for at least the first month of treatment. Once the patients have reported only mild symptoms of any mental disorders, self-harming thoughts or cravings for at least three surveys in a row, Vista extends the recommended update frequency to every other week. Regardless of Vista’s recommendations, the frequency and timing of the update survey requests is completely at the discretion of the treatment provider, who can request an update survey for a particular patient at any time.
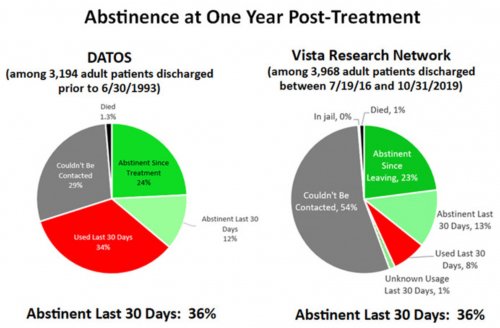
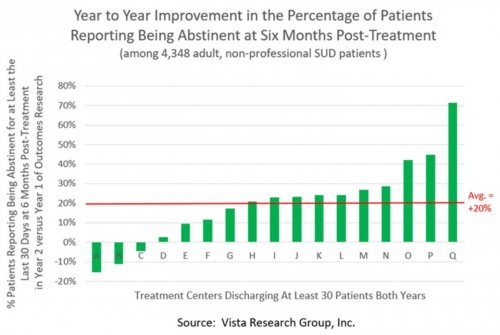
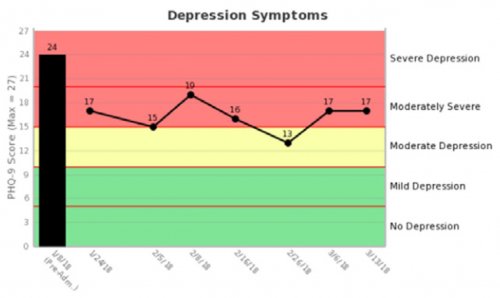
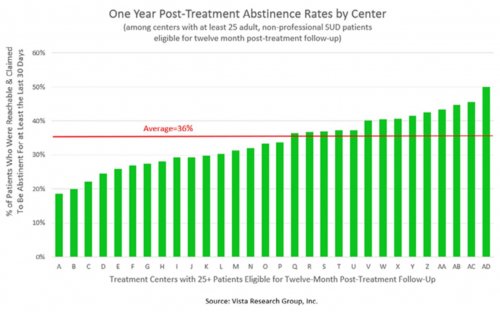
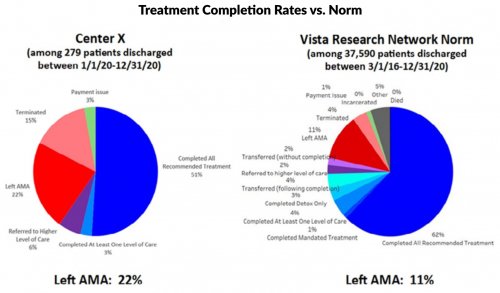
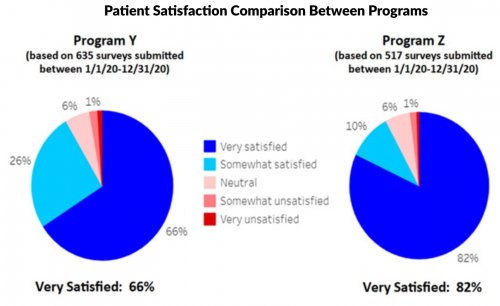
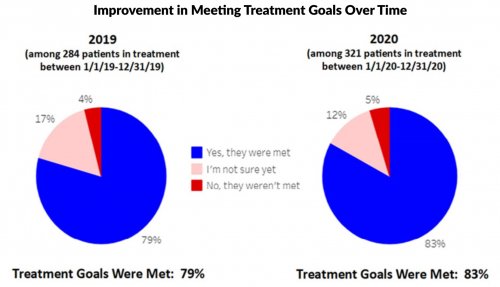
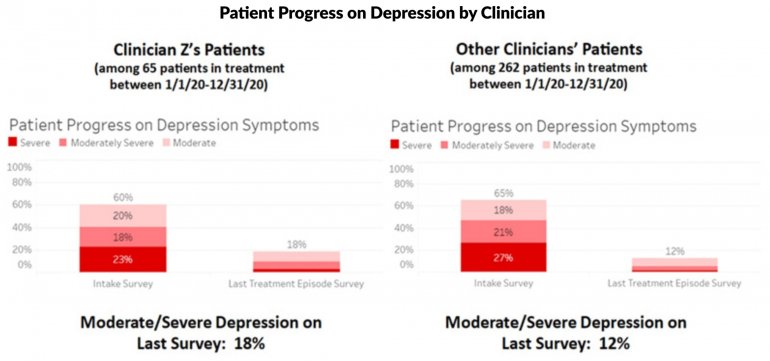
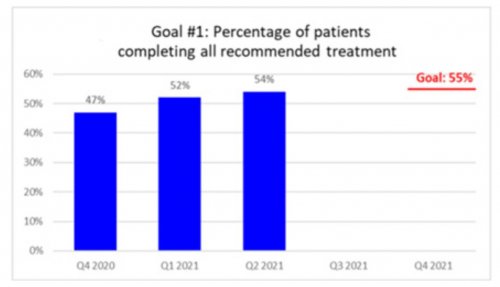
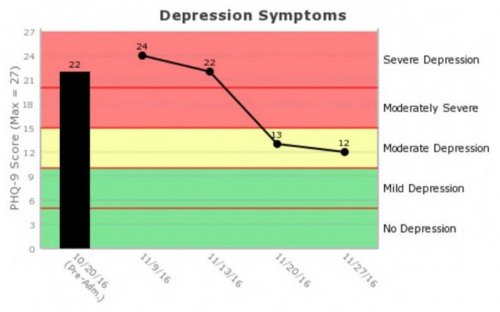
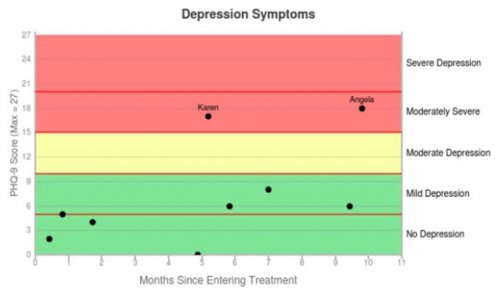
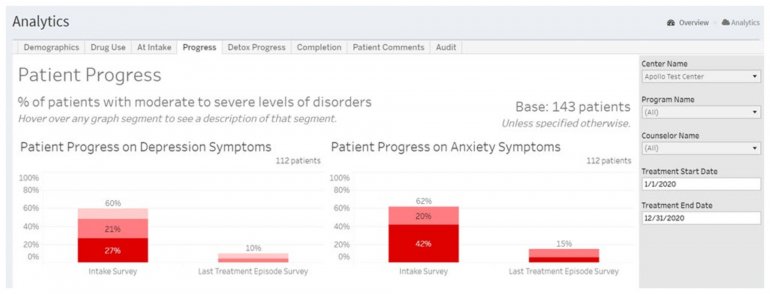
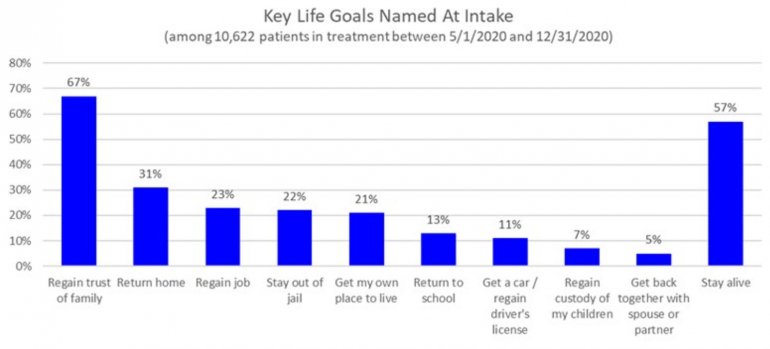
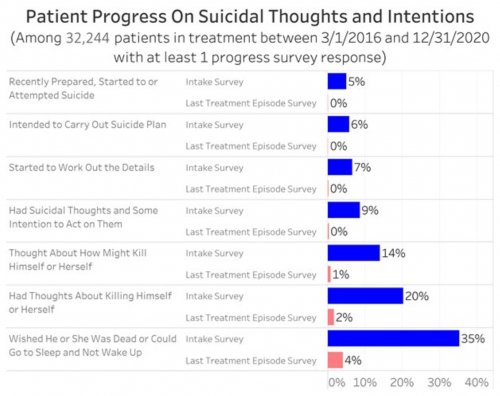
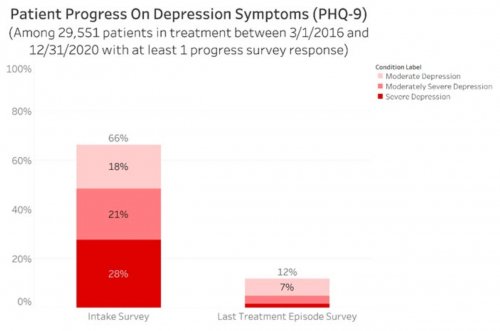
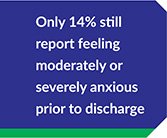
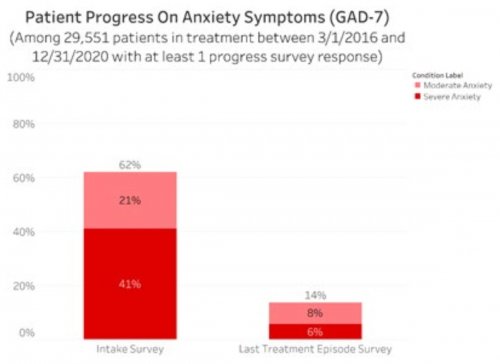
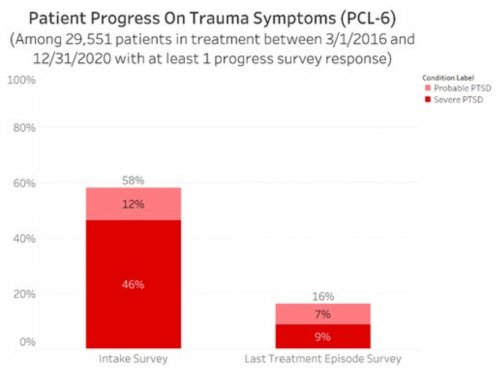
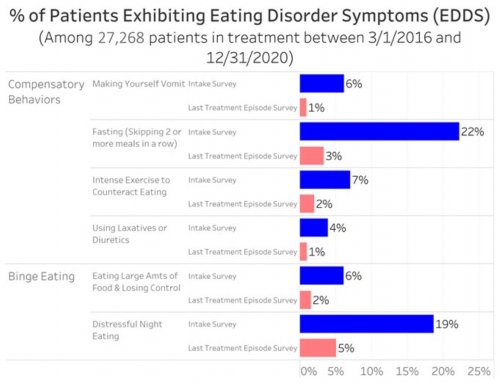
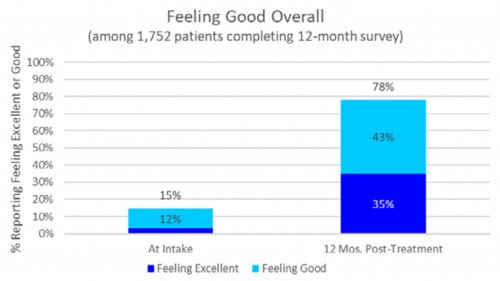
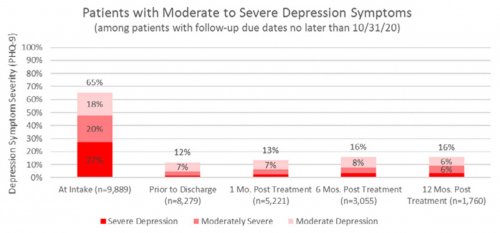
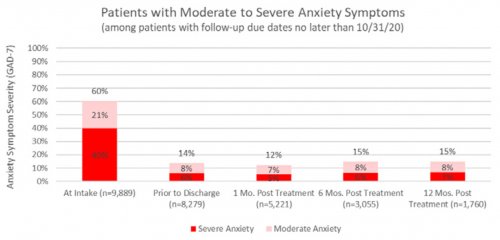
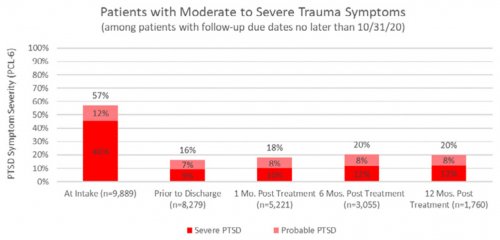
All data is shared with clinicians in real-time in easy-to-understand graphs such as the following: